CEJ:闡明晶體缺陷和熱處理對鋯基金屬有機(jī)框架的二氧化碳捕獲性能的影響
發(fā)布日期:2023-12-08 來源:貝士德儀器

第一作者:何山(汕頭大學(xué),22屆化學(xué)專業(yè)碩士研究生)、李凌簫(汕頭大學(xué),23屆材料科學(xué)與工程專業(yè)本科生)
通訊作者:陳曉嫻、周浩龍、
第一單位:化學(xué)與精細(xì)化工廣東省實(shí)驗(yàn)室、汕頭大學(xué)
論文DOI:https://doi.org/10.1016/j.cej.2023.147605
文章亮點(diǎn)
1.成功制備了無缺陷及富含缺陷的UiO-66型MOF。
2.闡明了缺陷對CO2捕獲性能的影響機(jī)理。
3.說明了熱處理活化/再生條件對CO2捕獲性能的重要性。
4.強(qiáng)調(diào)了MOF局部精細(xì)結(jié)構(gòu)的識別對其CO2捕獲性能評價(jià)的必要性。
摘要詳文
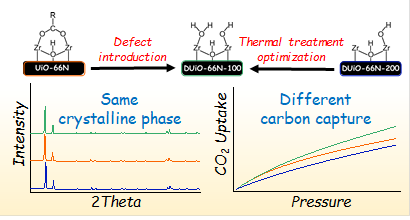
Graphical abstract
研究引入
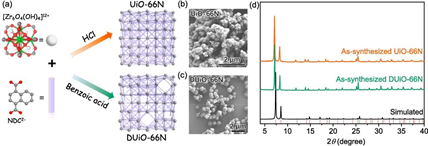
Fig. 1. Synthesis and characterization of UiO-66N and DUiO-66N. (a) Synthetic routes and simplified structural view. (b, c) SEM images. (d) PXRD patten comparison.
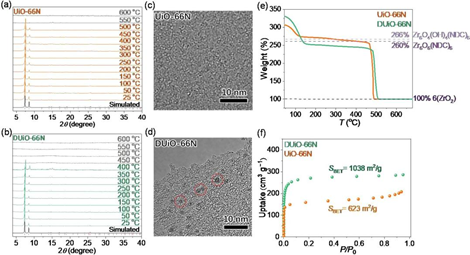
Fig. 2. Evidence for the introduction of crystalline defects. (a, b) VTPXRD of UiO-66N and DUiO-66N. (c, d) TEM images of the crystal edge of UiO-66N and DUiO-66N. (e) TGA curves of UiO-66N and DUiO-66N under oxygen atmosphere. (f) N2 sorption isotherms at 77 K of UiO-66N and DUiO-66N.
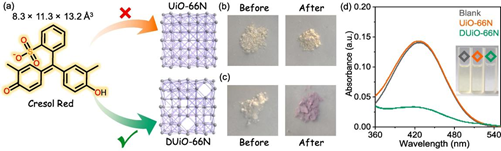
Fig. 3. The dye sorption experiment. (a) Structural view of the dye molecule. (b, c) Photographs of UiO-66N and DUiO-66N before and after the dye adsorption experiments. (d) Photographs and UV–vis spectra of the dye solutions. Blank: black; UiO-66N: orange; DUiO-66N: green.
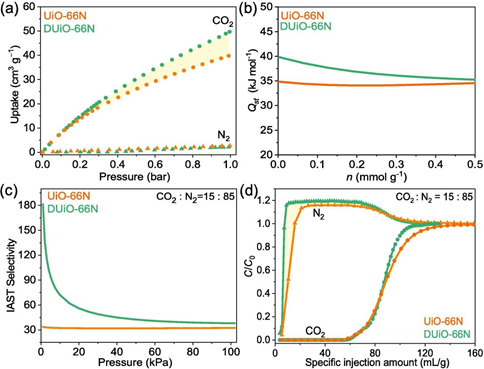
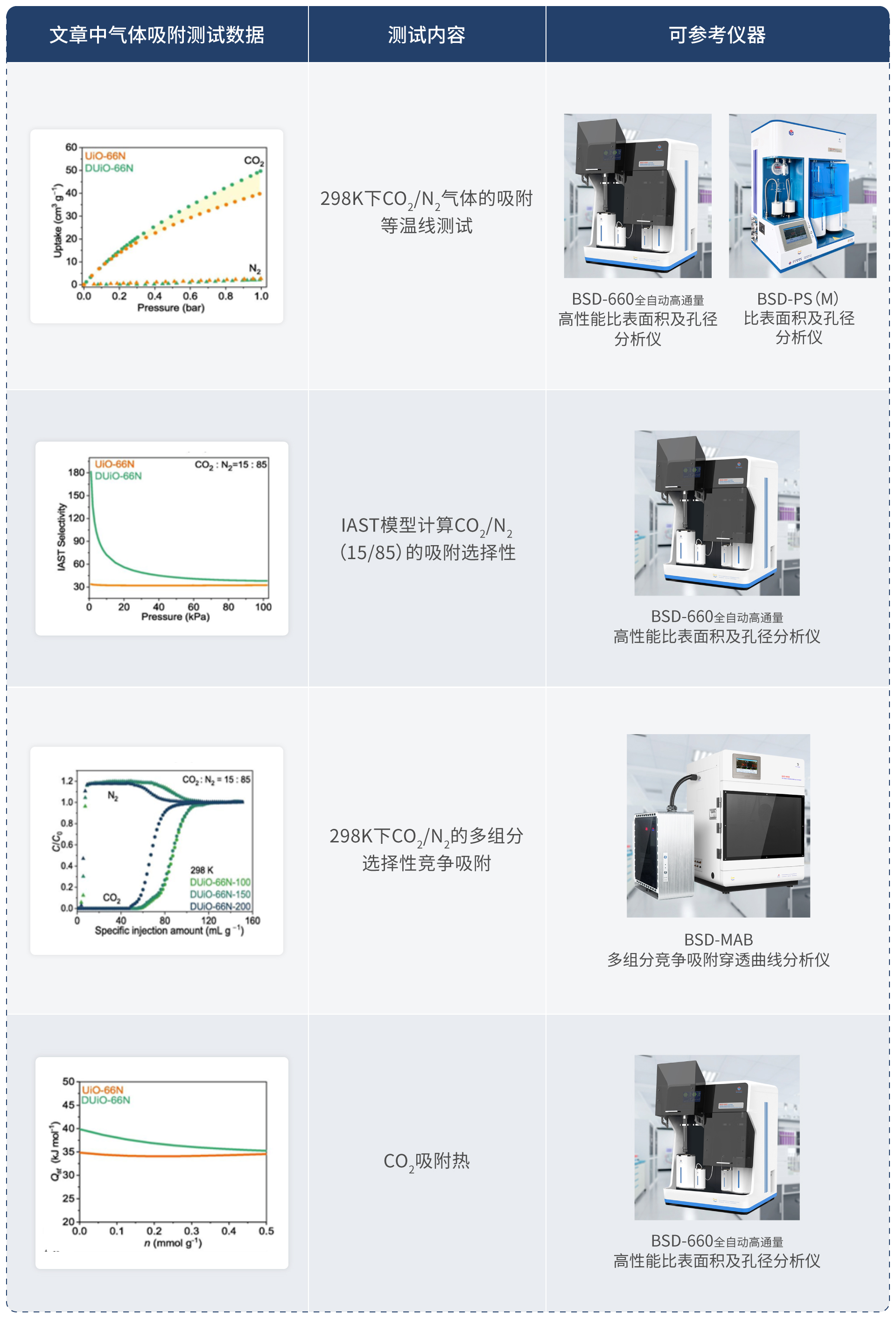
Fig. 4. The carbon capture performance comparison of UiO-66N and DUiO-66N. (a) CO2 and N2 sorption isotherms at 298 K. (b) CO2 adsorption enthalpy plots. (c) IAST selectivity plots. (d) Column breakthrough behaviors. UiO-66N: orange; DUiO-66N: green.
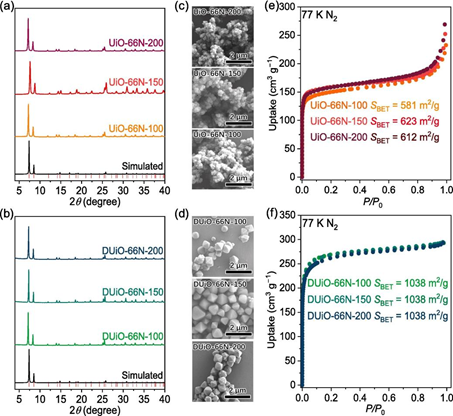
Fig. 5. Characterization of UiO-66N-X and DUiO-66N-X. (a, b) PXRD. (c, d) SEM images. (e, f) N2 sorption isotherms at 77 K.
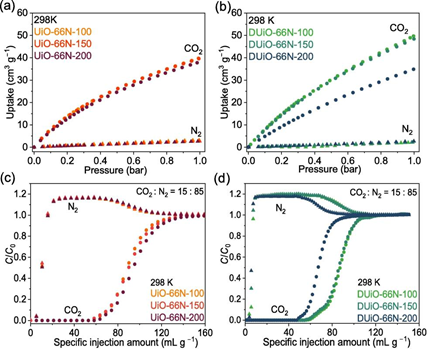
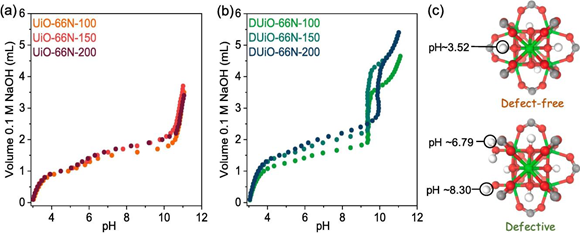
Fig. 7. Potentiometric acid–base titration. (a, b) Potentiometric acid–base titration curves of UiO-66N-X and DUiO-66N-X. (c) Calculated pKa values for the intact defect-free and low connected defective clusters.
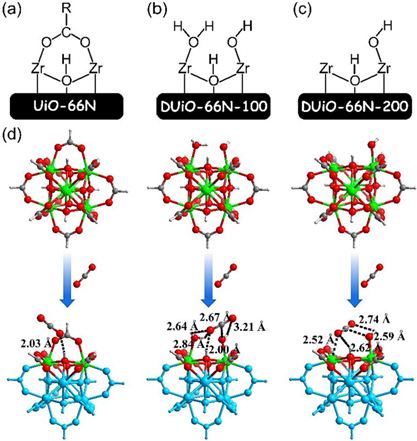
Fig. 8. Computed optimum structures for CO2 interactions with UiO-66N-X and DUiO-66N-X. (a-c) Schematic representation of the Zr-cluster structure models used for DFT calculation. (d) Structural view of binding geometries of the three Zr clusters for CO2.
文章結(jié)論
貝士德 吸附表征 全系列測試方案

1、填寫《在線送樣單》
2、測樣、送檢咨詢:楊老師13810512843(同微信)



4.png)




