CEJ:闡明晶體缺陷和熱處理對鋯基金屬有機框架的二氧化碳捕獲性能的影響
發(fā)布日期:2023-12-08 來源:貝士德儀器

第一作者:何山(汕頭大學,22屆化學專業(yè)碩士研究生)、李凌簫(汕頭大學,23屆材料科學與工程專業(yè)本科生)
通訊作者:陳曉嫻、周浩龍、
第一單位:化學與精細化工廣東省實驗室、汕頭大學
論文DOI:https://doi.org/10.1016/j.cej.2023.147605
文章亮點
1.成功制備了無缺陷及富含缺陷的UiO-66型MOF。
2.闡明了缺陷對CO2捕獲性能的影響機理。
3.說明了熱處理活化/再生條件對CO2捕獲性能的重要性。
4.強調(diào)了MOF局部精細結(jié)構(gòu)的識別對其CO2捕獲性能評價的必要性。
摘要詳文
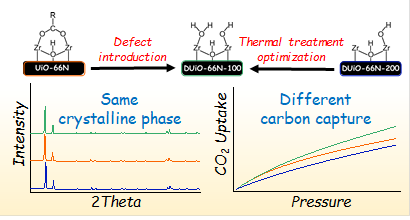
Graphical abstract
研究引入
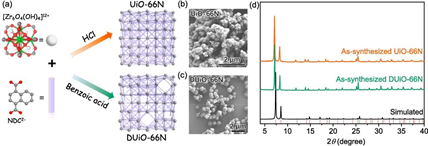
Fig. 1. Synthesis and characterization of UiO-66N and DUiO-66N. (a) Synthetic routes and simplified structural view. (b, c) SEM images. (d) PXRD patten comparison.
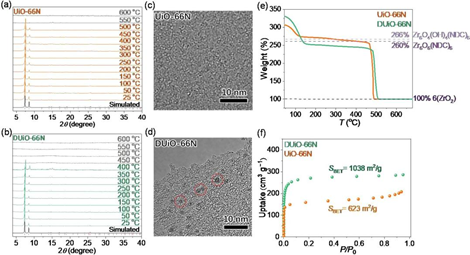
Fig. 2. Evidence for the introduction of crystalline defects. (a, b) VTPXRD of UiO-66N and DUiO-66N. (c, d) TEM images of the crystal edge of UiO-66N and DUiO-66N. (e) TGA curves of UiO-66N and DUiO-66N under oxygen atmosphere. (f) N2 sorption isotherms at 77 K of UiO-66N and DUiO-66N.
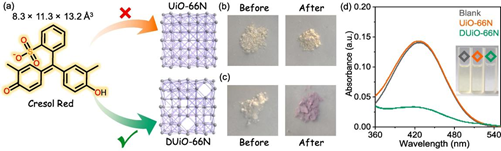
Fig. 3. The dye sorption experiment. (a) Structural view of the dye molecule. (b, c) Photographs of UiO-66N and DUiO-66N before and after the dye adsorption experiments. (d) Photographs and UV–vis spectra of the dye solutions. Blank: black; UiO-66N: orange; DUiO-66N: green.
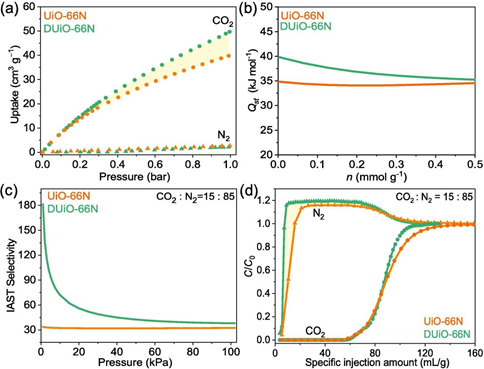
Fig. 4. The carbon capture performance comparison of UiO-66N and DUiO-66N. (a) CO2 and N2 sorption isotherms at 298 K. (b) CO2 adsorption enthalpy plots. (c) IAST selectivity plots. (d) Column breakthrough behaviors. UiO-66N: orange; DUiO-66N: green.
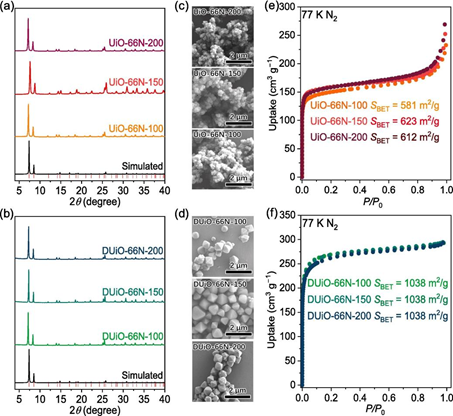
Fig. 5. Characterization of UiO-66N-X and DUiO-66N-X. (a, b) PXRD. (c, d) SEM images. (e, f) N2 sorption isotherms at 77 K.
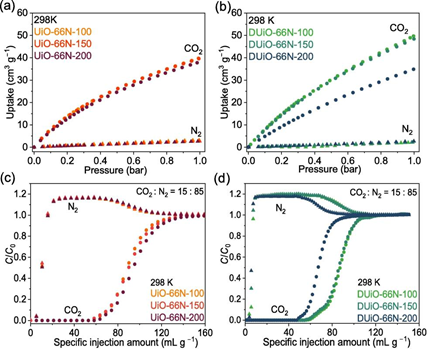
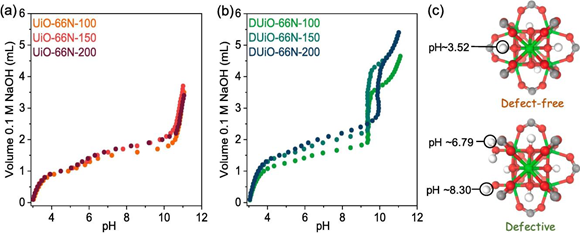
Fig. 7. Potentiometric acid–base titration. (a, b) Potentiometric acid–base titration curves of UiO-66N-X and DUiO-66N-X. (c) Calculated pKa values for the intact defect-free and low connected defective clusters.
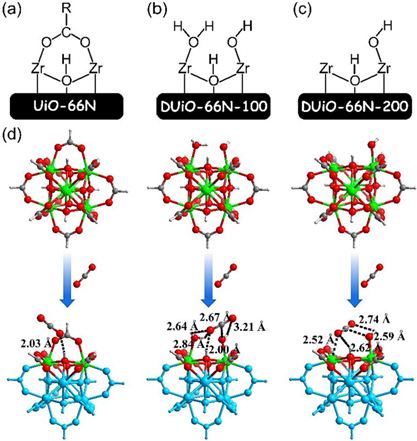
Fig. 8. Computed optimum structures for CO2 interactions with UiO-66N-X and DUiO-66N-X. (a-c) Schematic representation of the Zr-cluster structure models used for DFT calculation. (d) Structural view of binding geometries of the three Zr clusters for CO2.
文章結(jié)論
貝士德 吸附表征 全系列測試方案

1、填寫《在線送樣單》
2、測樣、送檢咨詢:楊老師13810512843(同微信)








